In the rapidly advancing world of digital medicine, the right information and strategies can make the difference between success and failure. With our holistic DiGA consulting, you are well equipped to master the many challenges and seize the many opportunities.
Table of Contents
DVG and DiGA fast track of the BfArM
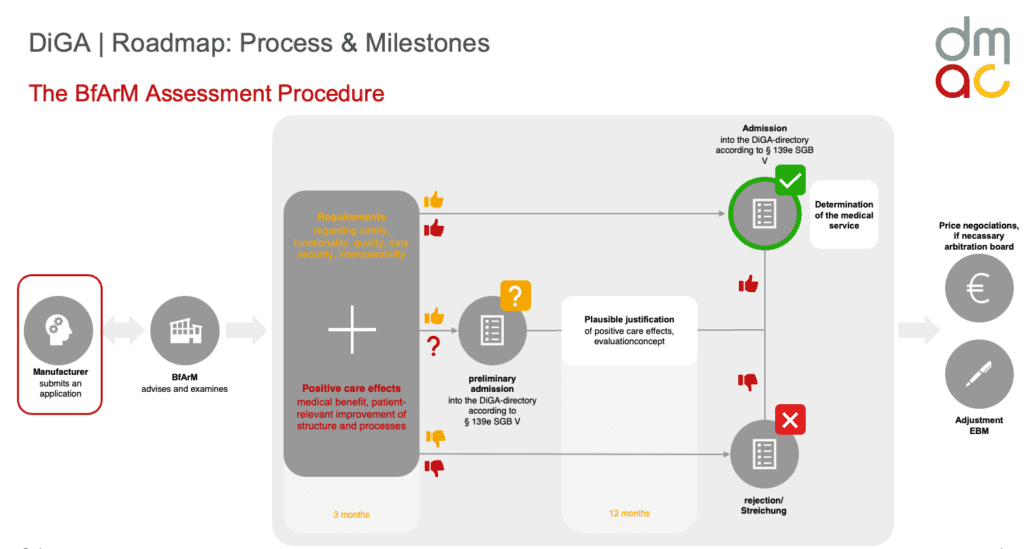
To ensure that DiGAs are of high quality and provide a proven benefit to patients, the DiGA fast-track procedure was introduced by the German Federal Institute for Drugs and Medical Devices (BfArM). This procedure offers manufacturers of digital health applications the opportunity to have their products tested quickly and, if the assessment is positive, to be included in the DiGA directory. An entry in this directory means that DiGA is reimbursed by the SHI and is thus accessible and financially covered for insured persons.
The DiGA fast-track process essentially comprises three building blocks:
- Testing of essential requirements: These include medical safety, privacy, quality and functionality of the application.
- Demonstration of positive care impact: the DiGA must show that it provides a medical benefit or a patient-relevant structured treatment process.
- Price negotiations: After inclusion in the DiGA directory, prices for the application are negotiated between the manufacturer and the GKV-Spitzenverband. After inclusion in the DiGA directory, the prices for the application are negotiated between the manufacturer and the GKV-Spitzenverband.
DiGA Criteria & DiGA Regulation (DiGAV)
However, not every digital health app is approved as a DiGA. To be approved as a DiGA, and thus eligible for reimbursement, digital health apps must meet a number of criteria.
These are the key requirements and aspects of the DiGA regulation:
- Medical device status: a DiGA must be classified as a Class I or IIa medical device under the Medical Device Regulation (MDR). This proves that it meets the general safety requirements for medical devices.
- Data protection and data security: The DiGA must comply with the requirements of the EU General Data Protection Regulation (GDPR) and the German Federal Data Protection Act (BDSG). They must also demonstrate that data is transmitted, stored and processed securely.
- Positive supply effects: The application should have a proven benefit for the patient, whether through symptom improvement, disease progression or even improved self-management skills of the patient.
- No adverse effects: The application must be safe in the medical sense. DiGAs must not harm the patient or discourage other necessary treatments.
- Quality management system: manufacturers must have implemented a quality management system according to DIN EN ISO 13485 for the design, development, production and distribution of their DiGA.
- Interoperability and usability: Applications should be technically sound, interoperable and compatible with other digital healthcare systems. The user interface should be understandable and patient-friendly.
- Scientific evaluation: the DiGA must be evaluated for efficacy and safety through clinical trials or other scientific methods.
- Documentation: manufacturers must provide comprehensive documentation including an evaluation concept, a data and IT security concept, and quality assurance documentation.
- Price indication: The DiGA must be provided with a price that is negotiated with the GKV-Spitzenverband as part of the reimbursement procedure.
- Neutral and advertising-free content: DiGAs must not contain advertising for other products or services and should be neutral in their communication of information.
Evaluation concept and study design
Conducting clinical studies
Pricing for DiGA
These are the key aspects of pricing for DiGAs:
- Preliminary reimbursement amount: after inclusion in the DiGA list, a preliminary reimbursement amount is initially reimbursed. This is equal to the manufacturer’s stated retail price, but cannot exceed a certain maximum amount.
- Price Negotiations: Price negotiations between the manufacturer and the GKV-Spitzenverband begin within one year of inclusion in the list. Negotiations are based on the proven benefit of the DiGA, the costs of comparable therapy alternatives, and other economic aspects.
- Basis for negotiations: During price negotiations, the positive supply effect of the DiGA is considered a key factor. For example, if a DiGA has been shown to reduce treatment duration or have other positive economic effects, this may influence the price.
- Duration of the price agreement: once agreed, prices are usually valid for a certain period of time. Thereafter, they may be renegotiated, particularly if the benefits of the DiGA or market conditions change.
- Rebate contracts: In addition to standardized price negotiations, manufacturers can also enter into individual rebate contracts with individual health insurers.
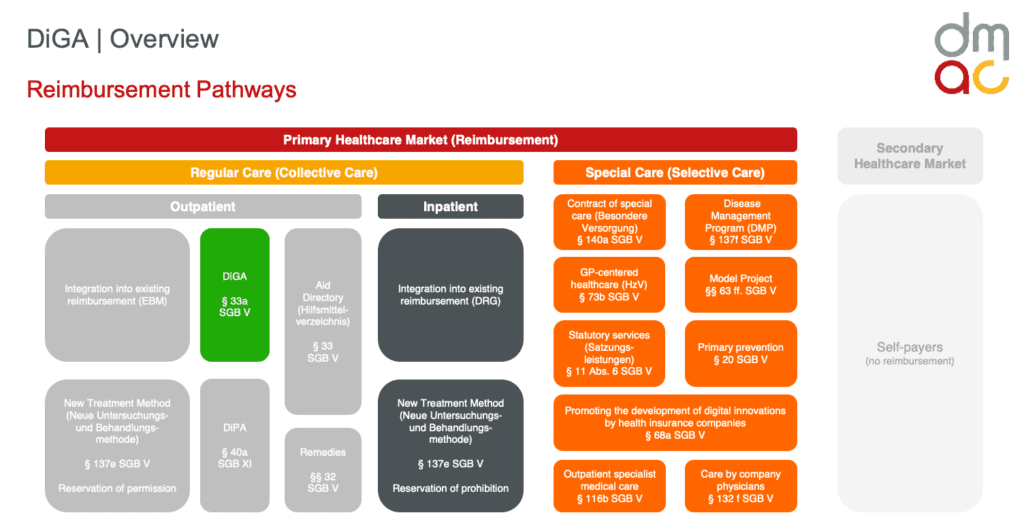
Other reimbursement options besides DiGA Fast Track
In addition to DiGA Fast Track, there are other reimbursement options available in the German healthcare market for various healthcare products and services. Some of these reimbursement options are:
- Digital care applications (DiPA): Digital care applications can include a variety of tools and programs that aim to support patient care, improve communication between caregivers and patients, or make administrative tasks in the care sector more efficient. DiPAs are currently reimbursable only for ambulatory care. Entitlement to a DiPA and any necessary supplementary care service is limited to €50 per month. The benefit is available on application to the long-term care insurance fund if the DiPA is listed with the BfArM (Federal Institute for Drugs and Medical Devices).
- G-BA evaluation procedure for medicinal products: New medicinal products are evaluated with regard to their additional benefit compared to comparator therapies. Based on this evaluation, price negotiations are conducted between the pharmaceutical company and the GKV-Spitzenverband.
- List of medical aids: Medical aids (e.g. wheelchairs, hearing aids) can be included in this list so that they are reimbursed by the statutory health insurance.
- New examination and treatment methods (NUB): Medical procedures that are new and innovative can be evaluated via this procedure and potentially included in the benefits catalog of the statutory health insurance.
- Hospital Remuneration Act and DRG system: Hospital services in Germany are financed via the DRG system (Diagnosis Related Groups). New treatment methods or technologies can be integrated into this system and financed in this way.
- Selective contracts: Health insurance funds can enter into contracts directly with service providers (e.g., physicians, hospitals, nursing services) to offer specific services that go beyond the usual level. These can also involve innovative treatment methods or technologies.
- Health programs and preventive services: Some health insurers offer special health programs or preventive measures for their members that go beyond the standard benefits. The costs for these are borne by the respective health insurance funds.
- Innovation Fund: The Innovation Fund promotes new forms of health care and health care research. Projects that aim to innovatively improve care in the statutory health insurance system can be financed via this fund.
These are just a few of the many reimbursement pathways in the German healthcare market. It is important that manufacturers and providers of healthcare products and services familiarize themselves with the different pathways and requirements in order to choose the right strategy for their specific situation. Our consulting services provide you with an overview of alternative pathways and support you in the strategic alignment of your product.
Our DiGA consulting services
As an experienced partner in the field of Digital Health Applications (DiGA), we offer comprehensive consulting and support services for manufacturers entering the German healthcare market. Our goal is to successfully navigate your digital solution through the DiGA fast-track process and position it efficiently in the market.
Preliminary consultation
- Initial assessment of the potential of your digital health application with regard to the DiGA process.
- Identification of optimization opportunities and potential hurdles
Strategic Planning
- Development of an individual roadmap for the DiGA approval process.
- Advice on pricing strategies and market opportunities
Technical and functional advice
- Support in meeting the technical and functional requirements of the BfArM
- Advice on user-friendliness, interoperability and data security
Compliance and Regulatory
- Help with legal compliance, especially in the area of data protection
- Preparation and review of documentation for the BfArM
Support in the evaluation process
- Support during the entire fast track process
- Communication with the BfArM and other relevant stakeholders
Post-Marketing Consulting
- Support with market strategy following successful DiGA approval
- Monitoring and advising on changing regulatory requirements
Our Promise
Our in-depth industry knowledge and ongoing dialog with the relevant regulatory authorities mean that we are ideally positioned to guide DiGA manufacturers through the complex approval and market process. We focus on transparency, individual support and solutions that are tailored to your exact needs.
Want to learn more? Contact us today for a no-obligation consultation and get your digital health app on the road to success!